









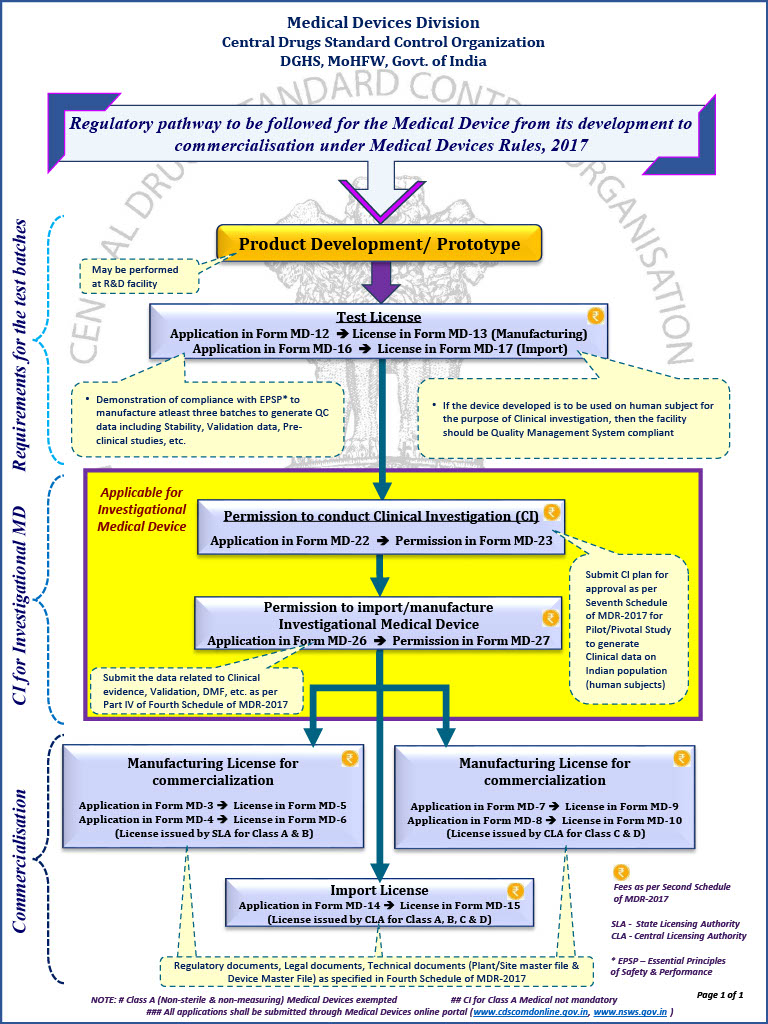
Regulatory pathway for Startups/Innovators for commercialization of Therapeutics, Vaccines, Medical Devices & IVDs
|
Frequently Asked Questions
CDSCO has released FAQs on Therapeutics, Vaccines, Medical Devices (MDs) & In-Vitro diagnostics (IVDs) to provide information on frequent questions and concerns in easy & understandable manner.
|