









Under the Guidance of NITI Aayog, Indian Council of Medical Research (ICMR) in partnership with Central Drug Standard Control Organization (CDSCO) aim to foster development of affordable and accessible indigenous Therapeutics, Vaccines, Medical Devices/ In-Vitro Diagnostics by providing strategic handholding support to MedTech innovators for clinical evaluation, regulatory facilitation and uptake of new products.
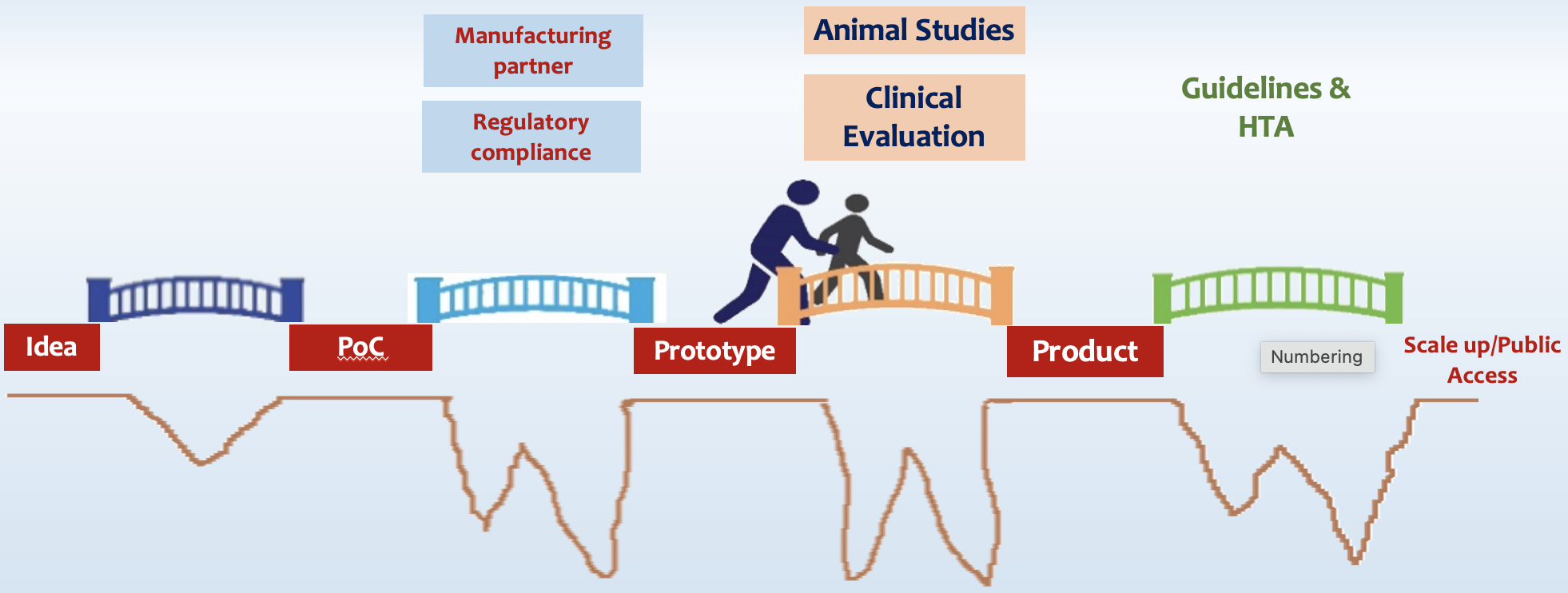
The journey of a new medtech product (e.g. therapeutics, vaccines, medical device or diagnostic) starts as an idea of an inventor who demonstrates its Proof-of-Concept (PoC) in a lab. Innovators and startups may be very good in their technical work, but find it hard to navigate the complex journey from ideation to product being used in clinical setting; and it is difficult to get timely and comprehensive guidance/facilitation. The difficulties faced by them are related to their lack of understanding and opportunities for regulatory requirements, testing and validation, industry grade production, animal studies, clinical evaluation/trials, technology assessment imperatives, among others. As a result, a large number of potentially effective MedTech products remain stuck at different stages of development pipeline, and do not see the light of the day.
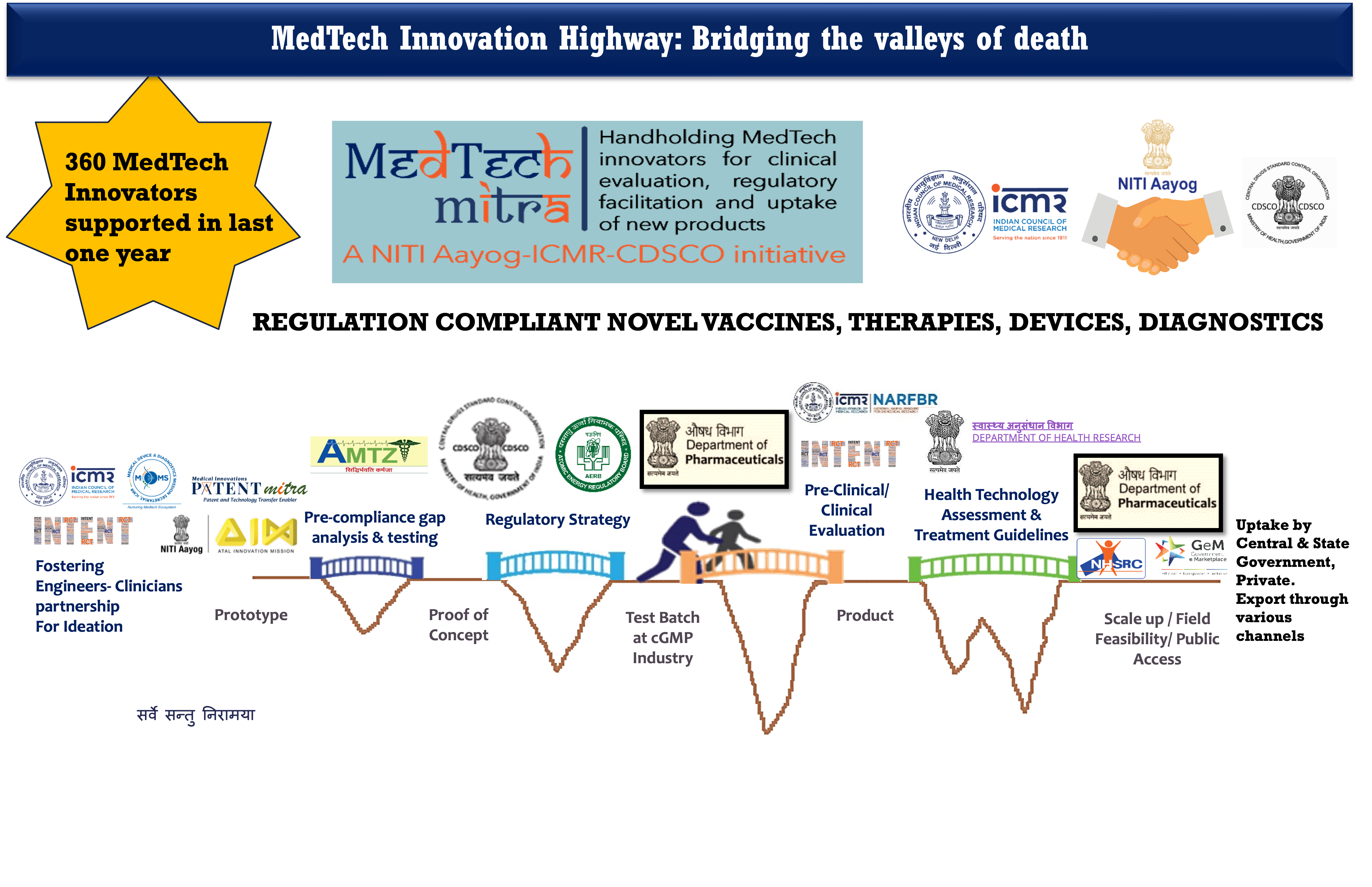
The Indian Council of Medical Research (ICMR) launched the MedTech Mitra initiative in partnership with the Central Drug Standards and Control Organization (CDSCO), under the guidance of NITI Aayog. A MedTech Mitra portal has been operationalized on ICMR website, coordinated by Division of Development Research at ICMR Hqrs. An applicant innovator / startup fills in online details of the product and stage of its development, and requests guidance. Atal Innovation Mission (AIM), Department of Pharmaceuticals, the INTENT network of medical research institutions, Kalam Institute of Health Technology, Department of Health Research-Health Technology assessment (HTAIn) and Centre for Guidelines are the core partners of this highway for development, validation, authorization and uptake of Make-in-India MedTech products.
MedTech Mitra Brochure |